
It has been almost four years since the world shut down due to COVID-19. It’s doubtful that anyone thought we would still be dealing with the effects of the virus in 2024. Life has been divided into “before COVID” and “after COVID.” Before COVID, the Centers for Medicare & Medicaid Services (CMS) was aggressively pursuing durable medical equipment (DME) suppliers with audits from all directions. When COVID hit and the public health emergency (PHE) was declared, most audits were paused.
In August 2020, CMS resumed its audit activities. During the PHE, CMS issued waivers that eased up on the clinical indications of many items with the goal of ensuring that patients had access to needed care. For this reason, audits did not focus on respiratory items such as oxygen and continuous positive airway pressure devices or other items with waived clinical indications. Instead, auditors focused on items such as surgical dressings, ostomy supplies, therapeutic shoes, parenteral and enteral nutrition, urological supplies and the provision of DME supplies in noncovered skilled nursing facility stays. In addition, auditors were still busy working through audits of the orthotics suppliers identified in Operation Brace Yourself.
Audit Activity
CMS audits have been moving forward at full speed, even before the official end of PHE on May 11, 2023. We are continuing to see audits of surgical dressings, as this is a complex product to bill. The 2021 Medicare Fee-for-Service Supplemental Improper Payment Report (Improper Payment Report) covering claims from July 1, 2019, through June 30, 2020, listed surgical dressings as having the highest improper payment rate at 69.7%, followed closely by therapeutic shoes with an error rate of 67.9%.
There has not been much change since that time, as the 2023 Improper Payment Report covering claims submitted between July 1, 2021, and June 30, 2022, shows the improper payment rate for surgical dressings is still 62.1%. Therapeutic shoes did show some improvement with an improper payment rate of 51.4%, but this is still significant. Due to the continued high error rates, you can count on continued audits of these products.
We have also been seeing increased audit activity with pneumatic compression devices (PCDs). PCDs were not listed in the top error rates for the 2021 Improper Payment Report, but in the 2023 report, PCDs have the second highest error rate behind oral cancer drugs at 78.9%. With an error rate that high, PCDs will be a focal point of audits. Other items identified in the 2023 Improper Payment Report for having high error rates include urological supplies, parenteral and enteral nutrition, manual wheelchairs and various orthoses. These items have all seen increased audit activity in the last year; as long as the error rates remain high, audit activity will continue.
Continuous Glucose Monitors
CMS has seen a drastic increase in the utilization of continuous glucose monitors (CGM). This is due, in part, to the relaxation of the clinical indications during the PHE. There has also been a change to the local coverage determination (LCD) that expanded coverage to include individuals who are treated with insulin or who have hypoglycemia and meet certain other requirements.
As a result of the increase in utilization, audits of CGMs have increased. We have seen audits of both PHE and post-PHE dates of service. For items such as CGMs where the clinical indications were waived during the PHE, CMS indicated it would be looking to primarily focus review on claims with dates of service outside the PHE, but that it can and will address dates of service during the PHE if it feels there are aberrant billing practices or potential fraud that need to be investigated. We have seen multiple payment suspensions and audits of CGM claims for dates of service during the PHE and would not be surprised to see this continue. We also expect to see increased audits of post-PHE dates of service due to the changes in the LCD.
TPE Audits
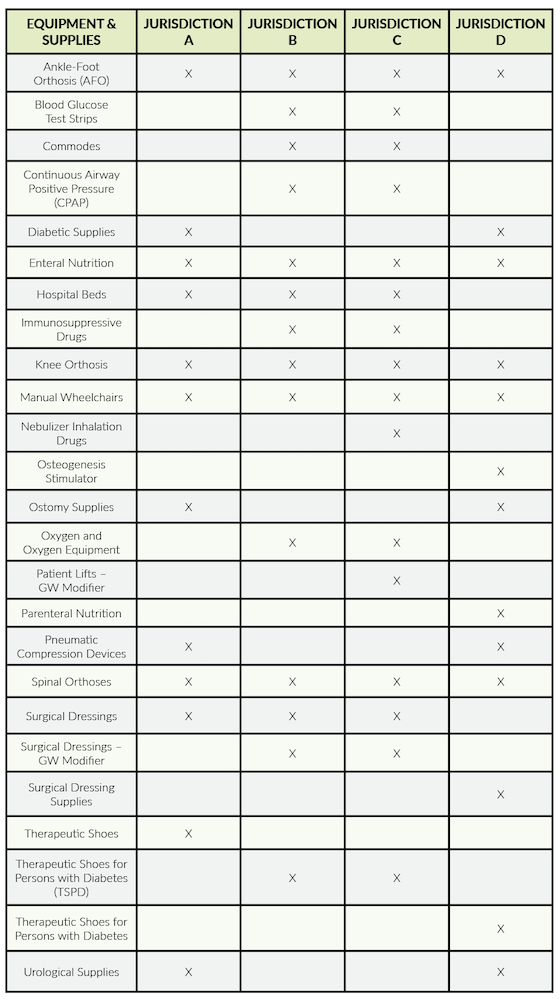
Targeted Probe and Educate (TPE) audits were some of the first audits resumed by CMS. The DME Medicare Administrative Contractors continue to conduct TPE audits. Both Noridian and CGS post audit results on their web pages detailing the items being audited under TPE and the results of those audits. The chart below lists the items being subjected to TPE audits by the various jurisdictions.
We recommend checking the websites for more details on these audits as they are the type most likely to be encountered by a DME supplier.
RAC Audits
The Region 5 Recovery Audit Contractor (RAC) is Performant Recovery, Inc., and it is responsible for auditing DME and home health/hospice claims. Before the RAC can audit an issue, it must have the issue approved by CMS. Performant posts CMS-approved issues on its webpage.
The RAC can perform either an automated review or a complex review. In an automated review, a determination can be made by looking at only claim data. There is no need to request additional documentation from the supplier. These types of audits can include denials for the same or similar or if services are covered under a Part A stay. The following automated reviews have been approved by CMS:
- Medical supplies billed from consolidated billing list during a home health episode; approved June 8, 2023.
- Canes, crutches, walkers reasonable useful lifetime (excessive units); approved May 1, 2023.
- CGM (excessive units); approved Sept. 8, 2020.
In a complex review, an additional documentation request is sent and the claims are reviewed before a determination is made. The following DME issues have been approved by CMS for a complex review:
- Hip orthosis; approved Sept. 15, 2023.
- Enteral and parenteral nutrition with dates of service after Sept. 5, 2021; approved Dec. 6, 2021.
- CGMs; approved Sept. 8, 2020.
Supplemental Medical Review Audits
In addition to RAC audits, CMS also contracts with Noridian Healthcare Solutions as the Supplemental Medical Review Contractor (SMRC). The SMRC conducts audits of items identified through CMS data analysis conducted by the Comprehensive Error Rate Testing (CERT) contractor or other agencies. The SMRC may also conduct audits as requested by CMS to protect the Medicare Trust Fund. A supplier can also find information on an SMRC webpage about most projects being worked on by the SMRC. CMS may request some projects not be listed.
The only current DME project listed by the SMRC is a review of lumbar-sacral orthoses. According to the CERT report, high error rates were found in 2021 and 2022, with error rates of 44.2% and 51.7%, respectively. The dates of service covered by the SMRC project are Jan. 1, 2021, through Dec. 31, 2022. Orthoses of all types continue to be of interest to all auditors, and the SMRC is no exception. Suppliers providing orthoses should expect to be audited.
ALJ Appeals
It should be noted that the administrative law judge (ALJ) appeals process is back on track, and while decisions are not quite meeting the 90-day deadline, it is no longer taking years for an ALJ appeal to be heard. These appeals are generally heard within a few months. To clear the backlog, the Office of Medicare Hearings and Appeals has made a significant investment in staff and resources. There are now 10 offices located around the country, with a satellite office in Denver. When the backlog has been fully cleared, these offices are not going to be closed; instead, this increased capacity is likely to mean more audits.
In general, suppliers can expect to see an increase in overall audit activity. These audits will certainly cover the items mentioned above, but suppliers should keep in mind that with the end of the PHE, oxygen and respiratory products are back in play. Due to the volume of claims at issue, these items will definitely see some sort of audit activity.
The key for most suppliers is to stay informed. Most CMS auditors will post information on their web pages about the types of audits being conducted and the results. By being knowledgeable, a supplier can be proactive and perform self-audits often—identifying errors before a CMS contractor comes knocking.
