WASHINGTON—The Centers for Medicare & Medicaid Services (CMS) posted its final notice for Transitional Coverage for Emerging Technologies (TCET) on Aug. 7. The TCET Pathway helps people with Medicare access the latest medical advances, enables doctors and other clinicians to provide the best care for their patients and benefits manufacturers who create innovative technologies.
CMS issued a final procedural notice outlining a Medicare coverage pathway to achieve more timely and predictable access to certain new medical technologies for people with Medicare. The new TCET pathway for certain Food & Drug Administration (FDA)-designated Breakthrough Devices increases the number of NCDs that CMS will conduct per year and supports both improved patient care and innovation by providing a clear, transparent and consistent coverage process while maintaining robust safeguards for the Medicare population. CMS said it anticipates accepting up to five TCET candidates per year and, for technologies accepted into and continuing in the TCET pathway, CMS said its goal is to finalize a national coverage determination (NCD) within six months after FDA market authorization.
"CMS is committed to making sure people with Medicare have access to medical advancements that improve health outcomes and enhance health quality," CMS said in the notice. "With support from policymakers, trust from patients and providers, and meaningful collaboration with manufacturers, CMS aims to improve the care and quality of life for people with Medicare while enhancing and encouraging innovation."
Below is what was outlined in the notice:
TCET Pathway at a Glance
The TCET pathway is intended to balance multiple considerations when making Medicare coverage determinations: (1) facilitating early, predictable and safer access for people with Medicare to new technologies; (2) reducing uncertainty about coverage by evaluating early the potential benefits and harms of technologies with manufacturers; and (3) encouraging evidence development if notable evidence gaps exist for coverage purposes.
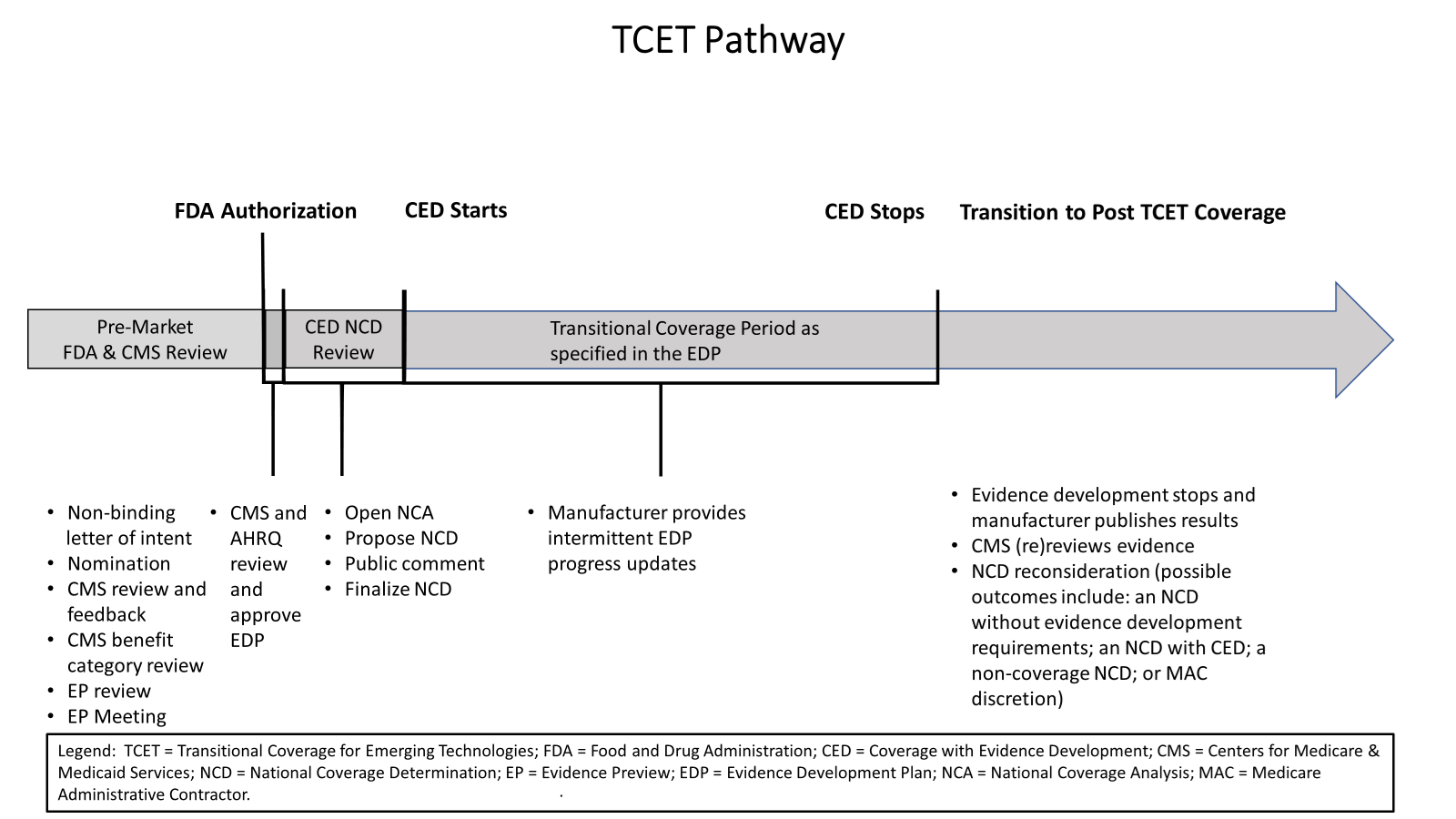
How TCET Works
The TCET pathway uses current national coverage determination (NCD) and coverage with evidence development (CED) processes to expedite Medicare coverage of certain FDA-designated Breakthrough Devices. TCET balances providing people with Medicare access to the latest medical innovations while ensuring these new technologies are appropriate and beneficial to the Medicare population based on data and available evidence. In addition, TCET promotes patient-centered care by supporting evidence development so that people with Medicare, their caregivers and their doctors will have reliable information about the latest breakthrough technologies to make informed health care decisions. Finally, TCET gives device manufacturers what they have long asked for: a more efficient and transparent Medicare coverage review process that allows for enhanced communications between industry and CMS, clear evidence requirements and defined timelines for final coverage actions.
TCET is voluntary for manufacturers and aims to reduce uncertainty about coverage options through a pre-market evaluation of potential harms and benefits of technologies while identifying any important evidence gaps. Additionally, the TCET pathway includes an evidence development framework that provides manufacturers with opportunities for increased pre-market engagement with CMS and reduces manufacturer burden, with increased flexibility to address evidence gaps, to ultimately support Medicare coverage. Specifically, TCET allows for any evidence gaps to be addressed through fit-for-purpose studies with patients that reflect the Medicare population. A fit-for-purpose study is one where the study design, analysis plan and study data are appropriate for the question the study aims to answer. Furthermore, the TCET pathway will help coordinate Medicare benefit category determination, coding and payment reviews, further helping translate innovation into meaningful patient access.
When developing the pathway, CMS solicited extensive feedback from patient groups, medical professionals, device manufacturers, innovators and other Federal agencies. This feedback included requests for CMS to utilize a more agile, iterative evidence review process that considers fit-for-purpose study designs, including those that make secondary use of real-world data. CMS partnered with the Agency for Healthcare Research and Quality (AHRQ) to develop a comprehensive approach that incorporates greater flexibility into the CED paradigm and allows fit-for-purpose study designs. As the pathway is implemented, CMS will continue to engage with interested parties to ensure Medicare promotes access to emerging medical technologies while maintaining appropriate safeguards and rigorous evidence standards essential to the health of people with Medicare.
Evidence Development Under TCET
Evidence development to support Medicare coverage through the TCET pathway will be based upon a more transparent and predictable evidence-generation framework. In addition to the TCET procedural notice, CMS has finalized updated criteria in the CED guidance document based on the November 2022 AHRQ Report, and February 2023 Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) meeting as well as final National Coverage Analysis Evidence Review guidance to more clearly allow for fit-for-purpose study designs. CMS expects to publish more detailed fit-for-purpose guidance in the future. Additionally, CMS finalized the first in a series of guidance documents that review health outcomes and their clinically meaningful differences within priority therapeutic areas, beginning with knee osteoarthritis. CMS’ guidance documents can be accessed here.
Key components of the TCET pathway to facilitate evidence development include:
- An Evidence Preview, which is a focused literature review for a specific item or service, will provide early feedback on the strengths and weaknesses of the available evidence, including any evidence gaps. It is intended to inform CMS and manufacturers about the best available coverage options for an item or service and offers greater efficiency, predictability and transparency to manufacturers and CMS on the state of the evidence and any notable evidence gaps. CMS intends for Evidence Previews to be conducted by a contractor using standardized evidence grading, risk of bias assessment and applicability assessment. If an NCD is opened following the Evidence Preview, an evidence summary, including a disclosure of which contractor completed the review, will be posted with the tracking sheet on the CMS website for public comment.
- An Evidence Development Plan (EDP) will be developed by the manufacturer to address any evidence gaps identified in the Evidence Preview. EDPs may include traditional clinical study designs and/or fit-for-purpose study designs, including those that rely on secondary use of real-world data, consistent with applicable CMS guidance documents. The development of an EDP will include CMS-AHRQ collaboration to evaluate the EDP to ensure it meets established standards of scientific integrity and relevance to the Medicare population. Additionally, CMS will engage with the manufacturer to provide feedback and discuss any recommended refinements. Non-proprietary elements of the CMS and AHRQ-approved EDP will be made publicly available on the CMS website when a proposed NCD is posted.
Device Eligibility
Given the unique FDA criteria for Breakthrough designation status, the TCET pathway will apply to certain eligible FDA-designated Breakthrough Devices because this is the area with the most immediate need for a pathway like TCET.
Appropriate candidates for the TCET pathway would include those devices that are:
- FDA-designated Breakthrough Devices.
- Determined to be within a Medicare benefit category.
- Not already the subject of an existing Medicare NCD.
- Not otherwise excluded from coverage through law or regulation.
The Federal Food, Drug, and Cosmetic Act definition of medical device includes in vitro diagnostic (IVD) products, such as diagnostic laboratory tests. IVDs, including diagnostic laboratory tests, are a highly specific area of coverage policy development, and CMS has historically delegated review of many of these tests to specialized Medicare Administrative Contractors (MACs). CMS said it believes the majority of coverage determinations for IVDs granted FDA-designated Breakthrough Device status should continue to be made by the MACs through existing pathways and has prioritized the devices listed above for the TCET pathway given available resources.
Nominations for the TCET Pathway
Manufacturers of FDA-designated Breakthrough Devices may self-nominate to participate in the TCET pathway. Manufacturers may submit a non-binding letter of intent to nominate a potentially eligible device for the TCET pathway, approximately 18 to 24 months before anticipated FDA marketing authorization as determined by the manufacturer. The appropriate timeframe for manufacturers to submit TCET pathway nominations to CMS is approximately 12 months prior to when the manufacturer anticipates an FDA decision on a submission.
The notice outlines the information that manufacturers should include in the voluntary letter of intent and self-nomination packet. CMS will accept suitable TCET candidates quarterly. If a suitable nomination is not selected in the first review, it will be automatically considered in the subsequent quarter. Manufacturers will not need to resubmit to be considered in a subsequent quarter but can withdraw if they choose to do so. CMS’ consideration of nominations will include an initial meeting with the manufacturer to discuss the technology and answer any questions on the process. In addition, CMS will meet with FDA to help CMS gain a better understanding of the device and potential FDA review timing. As part of the nomination consideration process, the technology may undergo a benefit category review. Upon completion of CMS’ review of the nomination, CMS will notify the manufacturer by email whether the product is an appropriate candidate for the TCET pathway.
TCET & the NCD Process
If a device accepted into the TCET pathway receives FDA marketing authorization, CMS will initiate the NCD process by posting a tracking sheet following FDA market authorization (that is, the date the technology receives premarket approval (PMA); 510(k) clearance; or the granting of a De Novo request) pending a CMS and AHRQ-approved EDP (in cases where there are evidence gaps as identified in the Evidence Preview). The process for Medicare coverage under the TCET pathway would follow the NCD statutory timeframes in section 1862(l) of the Social Security Act (the Act). CMS’ goal is to finalize a TCET NCD within six months after FDA market authorization, it said.
Duration of Coverage Under the TCET Pathway
Coverage under the TCET NCD will continue only as long as needed to facilitate the timely generation of evidence that can inform patient and clinician decision-making. The duration of transitional coverage through the TCET pathway will be tied to the CMS- and AHRQ-approved EDP. The review date specified in the EDP will provide one additional year of coverage after study completion to allow manufacturers to complete their analysis, draft one or more reports and submit them for peer-reviewed publication. In general, CMS said it anticipates coverage under a TCET NCD may last for approximately five or more years as evidence is generated to address evidence gaps identified in the Evidence Preview and lead to a predictable, long-term Medicare coverage determination.
Transition to Post-TCET Coverage
CMS intends to conduct an updated evidence review within six calendar months of the review date specified in the EDP. Similar to the process used for the Evidence Preview, CMS intends to engage a third-party, neutral contractor to conduct the systematic literature review. The contractor will perform a qualitative evidence synthesis and compare those findings against the benchmarks for each outcome specified in the CMS and AHRQ-approved EDP that was developed to fulfill the original NCD requirements. CMS will conduct quality assurance on the contractor review. CMS will also review applicable practice guidelines and consensus statements and consider whether the conditions of coverage remain appropriate.
Based upon this assessment, when appropriate, CMS will open an NCD reconsideration by posting a decision that proposes one of the following outcomes: (1) an NCD without evidence development requirements; (2) an NCD with continued evidence development requirements; (3) a non-coverage NCD; or (4) rescinding the NCD, resulting in coverage decisions being made by MACs under section 1862(a)(1)(A) of the Act. Standard NCD processes and timelines will continue to apply, and following a 30-day public comment period, CMS will have 60 days to finalize the NCD reconsideration.
Coverage of Similar Devices
FDA market-authorized Breakthrough Devices are often followed by similar devices that other manufacturers develop. CMS believes it is important to let physicians and their patients make decisions about the best available treatment depending on the patient’s individual situation. NCDs are limited to particular items or services, but it is possible that more than one device could fall under the same NCD because it addresses the same indication. We recognize that some differences may exist for technologies in a class that may result in a distinct benefit/risk profile, and each will be evaluated on its own merit. In these instances, CMS will follow the existing NCD process detailed in section 1862(l) of the Act.
Changes from Proposed Notice to Final Notice
Based on CMS’ analysis of the topics raised during the public comment period, CMS made several changes between the proposed notice and final notice. A comprehensive list of changes is provided in the final notice. Notable changes include:
- CMS incorporates an opportunity for manufacturers to submit a non-binding letter of intent to nominate a potentially eligible device approximately 18 to 24 months before anticipated FDA marketing authorization.
- It clarified that when CMS is aware that manufacturers will likely pursue the TCET pathway for devices where appropriate clinical endpoints are uncertain, it may preemptively conduct a clinical endpoints’ review, and may convene a MEDCAC panel. CMS notes that submission of a non-binding letter of intent may avoid delays in TCET reviews.
- CMS has revised the timeframe for reviewing TCET nominations. It will review nominations on a quarterly basis.
- It states that if an NCD is opened, an evidence summary, including a disclosure of which contractor completed the review, will be posted with the tracking sheet on the CMS website for public comment.
- CMS said that EDPs should incorporate interim reporting to ensure adequate progress and timely completion. Interim reports should also disclose any meaningful changes to prespecified study protocols, which are essential to transparency.
- CMS expresses its intent to release soon the proposed factors it will use to prioritize TCET nominations to provide greater transparency, consistency and predictability. (In the meantime, it will prioritize TCET candidates based upon the language from the Aug. 7, 2013, Federal Register notice (78 FR 48164) stating that in the event it has a large volume of NCD requests for simultaneous review, it will prioritize these requests based on the magnitude of the potential impact on the Medicare program and its beneficiaries and staffing resources.)
- CMS indicates that some information on TCET devices will be added to the NCD Dashboard, including the number of devices in the TCET pathway, the date of nomination, the date of acceptance and the date the NCD process was initiated. This dashboard will be updated on an ongoing basis.
Next Steps for Manufacturers Interested in the TCET Pathway
If interested in the TCET pathway, manufacturers simply need to notify CMS of their interest in TCET electronically via the Coverage Center Web site using the “Contact Us” link at cms.gov/Medicare/Coverage/InfoExchange/contactus.html. To be considered for the first quarterly review, nominations will need to be submitted by Oct. 31, 2024. The deadlines for the next three quarterly review cycles are Jan. 31, 2025, April 30, 2025 and July 31, 2025.
For more information on the TCET pathway, visit federalregister.gov/public-inspection/2024-17603/medicare-program-transitional-coverage-for-emerging-technologies.
